Counterfeit Versions of Muro 128® (Sodium Chloride Hypertonicity Ophthalmic Ointment, 5%) and Muro 128® (Sodium Chloride Hypertonicity Ophthalmic Solution, 5%) Found in the United States
Bausch + Lomb is communicating with the U.S. Food and Drug Administration (FDA) and is working to alert the public that counterfeit versions of Muro 128® (Sodium Chloride Hypertonicity Ophthalmic Ointment, 5%) and Muro 128® (Sodium Chloride Hypertonicity Ophthalmic Solution, 5%) are being sold by unauthorized online retailers in the United States.
Authentic Muro 128® is a FDA monograph product that is generally recognized as safe and effective in providing temporary relief from corneal edema.
The safety or efficacy of these counterfeit products cannot be assured and they should not be used.
The manufacture, quality, storage and handling of these counterfeit products may not meet FDA requirements. Bausch + Lomb has received some adverse event (AE) reports associated with these counterfeit Muro 128 products.
Authentic Muro 128® is currently available in the following packaging configurations (see images below):
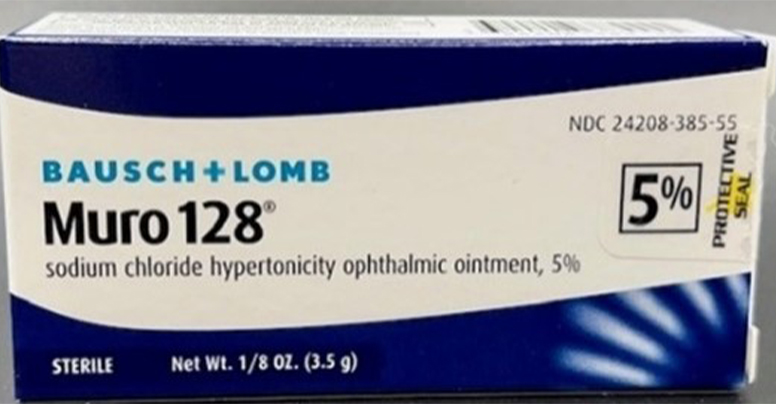
- Muro 128® (Sodium Chloride Hypertonicity Ophthalmic Ointment, 5%) – former brand packaging that is still on the market and have not expired
- Muro 128® (Sodium Chloride Hypertonicity Ophthalmic Ointment, 5%) – new brand packaging
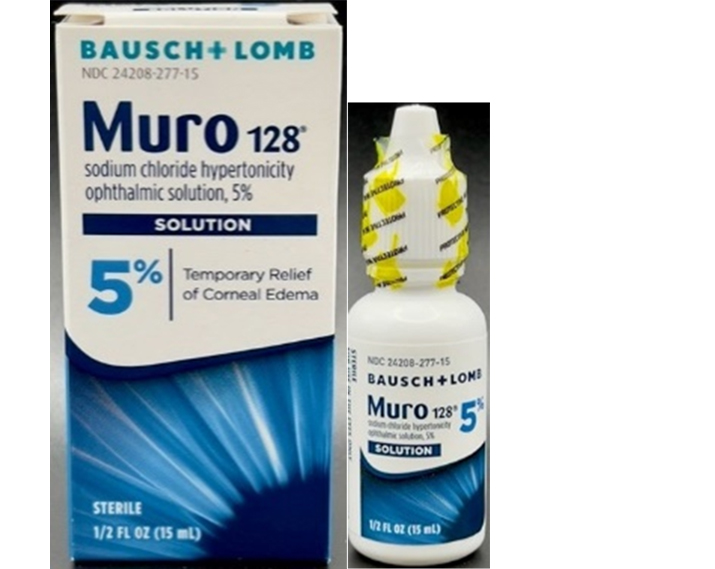
- Muro 128® (Sodium Chloride Hypertonicity Ophthalmic Solution, 5%)
How to Identify Counterfeit Product vs. Authentic Muro 128®
While there are similarities and differences between the counterfeit Muro 128 products and the authentic Muro 128® products, there are also several ways to confirm if a product is authentic Muro 128®.
The counterfeit products can be identified by one or more of the following:
Counterfeit Muro 128 - Ointment
- the outer carton and tube may not have a lot number or expiry date
- the outer carton may have typos in the drug facts section, and the word spacing may not be the same as authentic Muro 128® (Sodium Chloride Hypertonicity Ophthalmic Ointment, 5%) packaging
- the outer carton may display the active ingredient as “ointment chloride” instead of “sodium chloride”
- the outer carton may not include the correct trademarks; counterfeit uses a degree symbol (Muro 128°) vs. authentic registered mark (Muro 128®)
- the outer carton may list the product as an “ointment” but include a bottle instead of a tube
- the outer carton, if purchased as a twin pack, may not include the words “twin pack”
- the tube may not have a hole in the tip from where the contents are dispensed
- the tube may be made of a plastic-type material whereas the authentic Muro 128® (Sodium Chloride Hypertonicity Ophthalmic Ointment, 5%) tube is made of metal
- the tube may not have an authentic cap; counterfeit cap is narrower, has a rounded top and does not have vertical ridges from the bottom of the cap to the top whereas authentic Muro 128® (Sodium Chloride Hypertonicity Ophthalmic Ointment, 5%) is wider, has a flat top and vertical ridges that extend from the bottom of the cap to the top
Counterfeit Muro 128 - Solution
- the outer carton and bottle may list the product as an ointment
- the plastic wrap-around seal may be missing
- the solution bottle and cap may be different than authentic Muro 128® (Sodium Chloride Hypertonicity Ophthalmic Solution, 5%); counterfeit is wider, shorter and may come in a blue or white cap whereas authentic Muro 128® (Sodium Chloride Hypertonicity Ophthalmic Solution, 5%) is leaner, taller and white
|
Authentic Muro 128® (Sodium Chloride Hypertonicity Ophthalmic Ointment, 5%) |
Counterfeit Ointment |
|---|---|
|
Authentic Muro 128® is currently available in two ointment packaging configurations: Former Ointment Carton and Tube New Ointment Carton and Tube |
 
Areas circled above depict the following:
|
|
Authentic Muro 128® (Sodium Chloride Hypertonicity Ophthalmic Solution, 5%) |
Counterfeit Solution |
 |
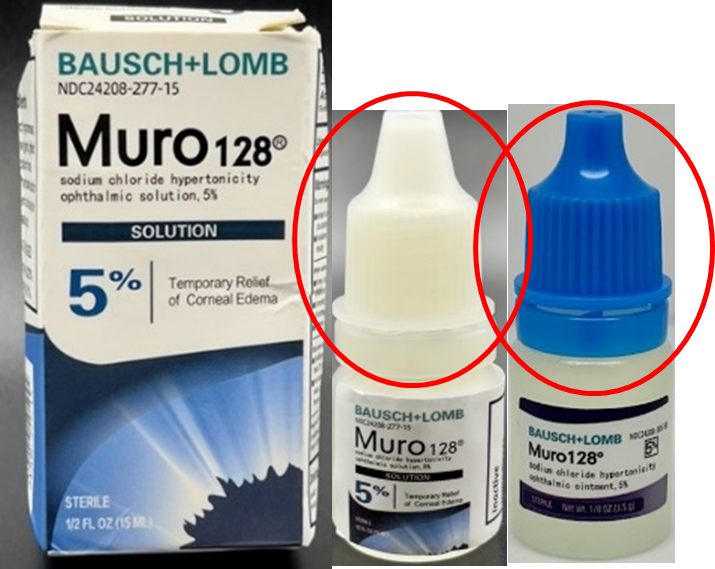
Areas circled above depict the following:
|
Health practitioners and consumers are advised to purchase authentic Muro 128® products through reliable retail sources and authorized distributors of Bausch + Lomb. Consumers are encouraged to check the product before using it to ensure its authenticity.
Medications purchased from foreign or unlicensed sources may be misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective, and/or unsafe. Medical practices that purchase and administer counterfeit, illegal and unapproved medications from unlicensed or foreign sources are putting patients’ health at risk, as patients may not be getting proper treatment.
To report counterfeit Muro 128 products or any suspected Bausch + Lomb counterfeit products, please call Bausch + Lomb customer care at 1-800-553-5340 Monday through Friday from 9 a.m. to 5 p.m. ET.
If an individual is experiencing any side effects that may be related to a Bausch + Lomb product or to the use of a counterfeit product, that person should immediately discontinue use and contact their health care provider, and is additionally encouraged to report the event to FDA’s MedWatch Safety Information and Adverse Event Reporting Program (1-800-FDA-1088 or www.fda.gov/medwatch).
Websites selling counterfeit and/or tampered medicines may be reported to the FDA. Suspected counterfeit products may be reported to FDA by calling the FDA’s Office of Criminal Investigations (OCI) at 1-800-551-3989 or a local OCI field office.